|
| ORCID Scopus Google Scholar Zotero IdRef |
| Scientific publications |
Main topics: Lanthanide Coordination Chemistry / Hexanuclear Complexes / Luminescence UV, vis., IR & Qy measurement
Filed patent application – n°1258525, France (September, 11th 2012)
FR2995316-A1 (2014-03-14) WO2014040917-A1 (2014-03-20)
PROCESS FOR MARKING AT LEAST ONE MATERIAL COMPRISING A SOLID OR LIQUID, ORGANIC OR MINERAL MATRIX, AND SAID CORRESPONDING MATERIAL
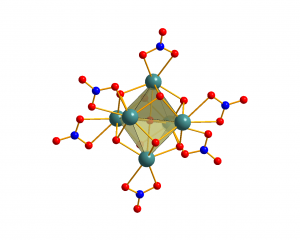
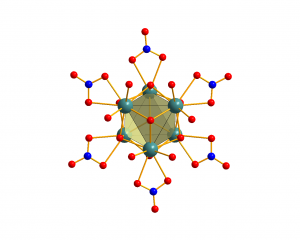
Hexanuclear complexes
Hexanuclear lanthanide complex [Ln6(µ6-O)(µ3-OH)8(NO3)6(H2O)12]2+ with Ln = Pr-Lu, Y
Using this compound as starting reagent we have obtained strongly luminescent coordination polymers (that we cannot obtain from simple salts)
The aim of this project is to obtain coordination polymers where polynuclear complexes would act as metallic centers _________________________________________________________________________
Luminescence
We study the luminescent properties of coordination polymers to:
- enhance the emitted light (for potential use as luminophores or phosphors)
- enhance the quantum yield Qy (which is quite different from the first point because their UV absorption varies greatly depending on the ligand being used)
- fine-tune the emitted color and excitation wavelength (playing on ligands and composition)
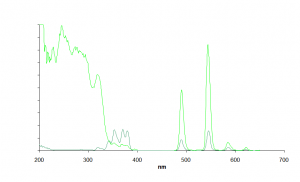
For instance:
These spectra correspond to two compounds, in dark green the starting complex and in light green the same complex functionalized so as to enhance its luminescence.
The excitation spectra are quite different: the functionalized compound shows a huge excitation band which rise above 300nm. This absorption band is due to the ligand being used (a benzene-polycarboxylate) which is able to transfer photons to the lanthanide and thus enhances its emissive properties. This well-known phenomena is called "antenna effect". _________________________________________________________________________
Quantum yield measurement
The Qy value gives the number of photons emitted by a sample divided by the number of photons absorbed by this same sample. We measure it in an integration sphere with a spectrophotometer.
We are often surprised by Qy values, because it is not an absolute measurement of the light emitted by the sample: the number of emitted photons is divided by the number of absorbed photons, not by that of the received ones. So, some compounds with a weak UV absorption may present a weak light emission but a high quantum yield.
In order to evaluate the absolute light emission properties of a luminophore, we use a fluxmeter to measure the UV light received on the surface (irradiance: W/cm²) and a lumenmeter to measure the visible light emitted by the surface (luminance: cd/cm²). After conversion of candelas in lumens (related to the 3D geometry of the measurement), the ratio of lm/W (i.e. luminous efficacy) can be compared with every other light emitting device (for example a commercial source of light).